A-level化学知识点梳理系列(十五)
点击量: 发布日期:2019-06-22 作者:四老师
有机化学(一):基本概念
有机化学是化学的一个非常重要的分支,也是A-level考试的重点考察对象。这一领域入门比较困难,和之前学到的化学知识没有太多联系,而且学完以后往往没有多少时间消化就要准备迎接考试。对于有机化学,大家一定要克服畏难情绪,勤加记忆,多加练习,就能在有机这一块拿到高分。
一、有机分子的表示方式
empirical formula
molecular formula
structural formula CH3CH=CH2
displayed formula
skeletal formula
二、官能团
三、有机分子的命名
1. Number the carbon atoms in the longest chain.
2. The numbering starts at the end that produces the lowest possible numbers in the name.
3. The hydrocarbon side-chain is named by adding –yl to the normal alkane stem. This type of group is called an alkyl group.
4. If there is more than one of the same alkyl side-chain, we indicate how many by inserting di, tri, tetra in front of its name.
5. If there is more than one type of alkyl side-chain, they are listed in alphabetical order.
6. In an alkene or alkyne, the longest carbon chain is numbered from the end that gives first carbon of the double/triple bond the lowest possible number.
四、有机分子中的化学键
sigma bond sp3 orbital hybridization
pi bond sp2 orbital hybridization
五、同分异构体
1、structural isomerism
ü functional group isomerism
alkene - cycloalkane
alcohol - ether
aldehyde - ketone
carboxylic acid – ester
ü chain isomerism
ü position isomerism
2、 stereoisomerism
ü cis-trans
ü optical
下面我们来看一道真题。
s12-qp12
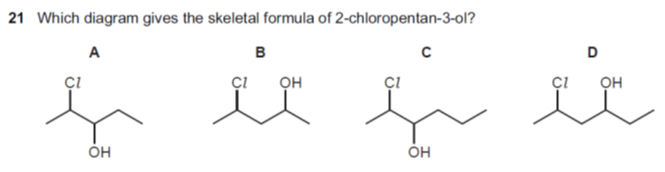
解析:这道题考察的是有机分子的命名法。从penta-可以看出有5个碳原子,排除C和D,再从2号碳原子连氯原子,3号碳原子连-OH可以判断正确答案是A。
有机化学是化学的一个非常重要的分支,也是A-level考试的重点考察对象。这一领域入门比较困难,和之前学到的化学知识没有太多联系,而且学完以后往往没有多少时间消化就要准备迎接考试。对于有机化学,大家一定要克服畏难情绪,勤加记忆,多加练习,就能在有机这一块拿到高分。
一、有机分子的表示方式
empirical formula
molecular formula
structural formula CH3CH=CH2
displayed formula
skeletal formula
二、官能团
| functional group | structure | general formula | name |
| alkene | ethene | ||
| arene | benzene | ||
| halogeno-alkane | chloromethane | ||
| alcohol | methanol | ||
| aldehyde | ethanal | ||
| ketone | propanone | ||
| carboxylic acid | ethanoic acid | ||
| ester | ethyl ethanoate | ||
| amine | methylamine | ||
| nitrile | ethanenitrile |
| no. of carbon atom | name of alkane |
| 1 | methane |
| 2 | ethane |
| 3 | propane |
| 4 | butane |
| 5 | pentane |
| 6 | hexane |
| 7 | heptane |
| 8 | octane |
| 9 | nonane |
| 10 | decane |
2. The numbering starts at the end that produces the lowest possible numbers in the name.
3. The hydrocarbon side-chain is named by adding –yl to the normal alkane stem. This type of group is called an alkyl group.
4. If there is more than one of the same alkyl side-chain, we indicate how many by inserting di, tri, tetra in front of its name.
5. If there is more than one type of alkyl side-chain, they are listed in alphabetical order.
6. In an alkene or alkyne, the longest carbon chain is numbered from the end that gives first carbon of the double/triple bond the lowest possible number.
四、有机分子中的化学键
sigma bond sp3 orbital hybridization
pi bond sp2 orbital hybridization
五、同分异构体
1、structural isomerism
ü functional group isomerism
alkene - cycloalkane
alcohol - ether
aldehyde - ketone
carboxylic acid – ester
ü chain isomerism
ü position isomerism
2、 stereoisomerism
ü cis-trans
ü optical
下面我们来看一道真题。
s12-qp12

解析:这道题考察的是有机分子的命名法。从penta-可以看出有5个碳原子,排除C和D,再从2号碳原子连氯原子,3号碳原子连-OH可以判断正确答案是A。
9年专注国际课程辅导培训
alevel、IB、AP、SAT2、IGCSE
上海天山总部:长宁区SOHO天山广场
上海浦东校区:浦东新区商城路618号良友大厦
上海七宝校区:闵行区新龙路399弄1号宝龙城
咨询热线:4006-321-553
广州校区:天河区体育西维多利广场
咨询热线:4006-321-556
宁波校区:宁波市鄞州区钱湖北路555号知识星球
咨询热线:4006-321-572




